Background: Cancer Stem Cells (CSCs)
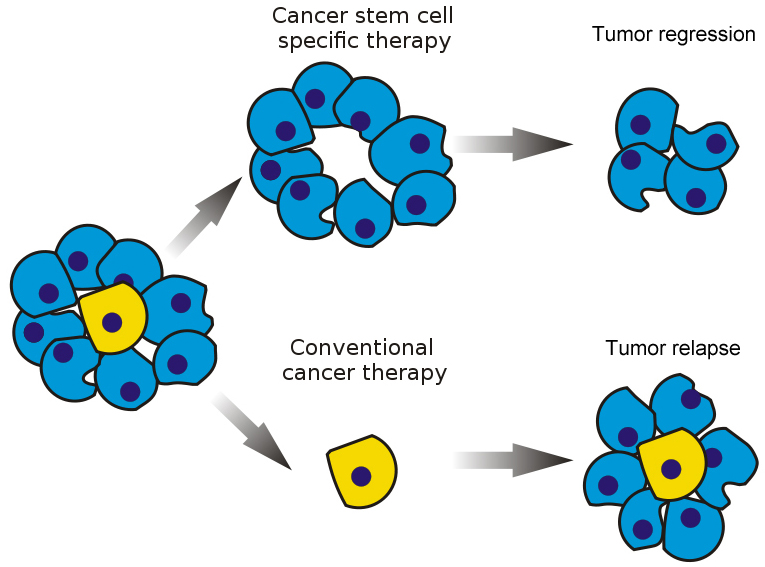
The CSC (or hierarchy) model suggests that tumors are composed of a heterogeneous group of cells that have arisen from stemlike precursors. As these tumor cells differentiate, they form a mixture of cells with different biological and enviro-genetic characteristics, forming a cellular hierarchy. At the apex of this hierarchy are cancer stem cells (CSCs), which serve as the source of newly formed tumor cells. Tumor relapse and spreading (metastasis) remain major obstacles for improving overall cancer survival, and may be due at least in part to the existence of cancer stem cells. CSCs are characterized by tumor-generating properties and the ability to self-renew, form differentiated offspring, and develop resistance to therapy. CSCs use many of the same signaling pathways that are found in normal stem cells, such as Wnt. Therapeutic targeting of both CSCs and bulk tumor populations may provide a strategy to suppress tumor regrowth. Recently, it has become apparent that Wnt signaling levels identify stem-like tumor cells that are responsible for fueling tumor growth . Development of agents that target critical steps in the Wnt pathway are complicated by cross-talk in molecular signaling and is a subject of current research (1).
Wnt/β-catenin signaling
Wingless-type Mouse Mammary Tumor Virus (WNT MMTV) integration site family, member 2, also known as WNT2, is a human gene. The Wnt pathway is a controller of gene transcription. The resulting proteins go on to define almost everything in the body, from tissue growth to brain function. Wnt signaling is currently considered a major contributor to CSC biology. Wnt signaling mechanisms regulate CSCs from initiation to metastasis. Increasing the number of receptors for Wnt signaling converts normal stem cells and differentiated cells into CSCs. Abnormally hyperactivated Wnt signaling allows entry into the early stages of metastasis and then facilitates persistent growth, invasion, migration, and homing of CSCs. Negative feed back of the Wnt signaling pathway then induces CSC dormancy, & subsequent hyperactivated Wnt signaling is central to re-initiation and colonization of metastasized sites. Although Wnt signaling inhibitors have been developed in multiple studies, their use is limited by the involvement of Wnt signaling in cell equilibrium (homeostasis) and development, leading to potential side effects. Target Genes of Wnt/β-Catenin Signaling. Wnt signaling regulates the expression of various genes through multiple pathways; β-catenin is a subunit of the cadherin protein complex and acts as an intracellular signal transducer in the Wnt signaling pathway. In the canonical pathway, low β-catenin expression is maintained through phosphorylation of serine and the addition of ubiquitin by protein degradation complexes (2).
In 2017 researchers at OncoMed Pharmaceuticals in Redwood City, California, tested two Wnt antagonists for clinical development: Vantictumab (anti-FZD) & ipafracept (FZD8-Fc). They found that these Wnt antagonists exhibit unique combinatorial anti-tumor activity with taxanes by potentiating mitotic cell death. The researchers used patient-derived xenograft (PDX) models as a preclinical drug discovery platform. Studies in pancreatic PDX models indicated that the combination of Wnt antagonists with taxane chemotherapy drugs was more effective in sensitizing cancer stem cells to the anti-tumor effect of taxanes (3).